Chapter Two: Why does CO2 Concentration Rise?
Tropical Forests do not fix CO2Trees produce hydrocarbons such as sugars, carbohydrates and cellulose from carbon dioxide and water using the energy of sunlight. As a byproduct of this process, trees release oxygen. This process is called photosynthesis and works in almost the exact opposite way in which we breathe in oxygen and exhale CO2. The process of photosynthesis is essential to the balance of the biosphere of the earth because the production of oxygen from CO2 recycles the CO2 that we produce and the oxygen that we consume through respiration. Because a large part of the world's trees are located in the Amazon Rainforests, the Amazon has been referred to as the "lungs of the Earth". But is this really true?
If the Amazon rainforest were continuously converting CO2 into hydrocarbons, then there would be a continuous accumulation of hydrocarbons in the forest. In other words, an endless growth of new trees and expansion of the forest area would occur. However, in reality, over the last thousand years, the area of the Amazon has been pretty much constant, and so has the size of its trees. Even the content of hydrocarbons in the soil layer has not changed. If the forest has not been expanding and the trees have not been getting bigger, then a continuous accumulation of hydrocarbons cannot be occurring. In fact, what we are seeing in recent years is actually a decrease in the number of trees and a shrinking of forest area due to the development and expansion of farmland and ranches that results in widespread deforestation.
Mature forests like the Amazon rainforest are not the "lungs of the Earth", because as a whole these forests do not release oxygen, nor do they fix CO2. The reason is as follows.
Through photosynthesis, CO2 absorbed by plants is broken down into oxygen and carbon using the energy of the Sun. While the oxygen is released back into the air, the carbon remains in the plant. This carbon is combined with water to form hydrocarbons, mainly in the form of cellulose, which is the main component of plant-life. However, while we usually think of plants as organisms that photosynthetically produce oxygen and hydrocarbons from CO2, they also must respire by consuming some of these hydrocarbons, just like animals. Approximately half of the hydrocarbons that are produced by photosynthesis are burnt or metabolized by the plants during respiration and exhaled as CO2. The energy produced by this respiration maintains the basic life functions of the plant in the same way as the basal metabolism of the human body keeps us going.
The remaining half of the hydrocarbons produced by photosynthesis goes into the growth of the trees as well as the production of leaves, flowers, and seeds. However, the leaves produced in spring will drop to the ground in autumn. The fallen leaves are consumed by insects and microorganisms. Just like in the case of plant respiration, the hydrocarbons in the leaves are metabolized into CO2 to provide these creatures with energy and nutrition. Even the part that is not metabolized and released instead as waste products are decomposed and oxidized into CO2 by smaller organisms or abiotic reactions in the soil. In other words, all of the carbon in the fallen leaves eventually return to CO2.
Consequently, the only net production of oxygen, fixation of CO2, and synthesis of hydrocarbons by a tree is the portion that goes into the growth of the tree itself. What happens to the trees in a mature forest when they grow?
Let us follow the life of a single tree. A tree fixes a large amount of CO2 to produce hydrocarbons during the process of germination and growth into a young tree. For many kinds of trees, the tree's fastest growth occurs about ten to twenty years after the tree's germination. In other words, the rate at which CO2 is fixed by the tree is the fastest at this point. After that, the tree's growth starts slowing down, nearly coming to a halt when it reaches old age. In due course, like all living things, the tree dies, falls down, and decays. Or it may be destroyed by lightning or a forest fire. Therefore, even the carbon that was fixed over the long period of the tree's life eventually returns to the atmosphere in the form of CO2.
A mature forest is a forest in which the above-mentioned cycle of carbon is steady. This is because a mature forest consists mainly of old trees with a few young trees growing in the clearings and open spaces left by those trees that died recently. The rapid CO2 fixation of the developing young trees is canceled out by the decay of the dead fallen trees. Therefore, taken as a whole, a mature forest remains constant in its size, for there is no excess of CO2 fixation, oxygen production, or production of hydrocarbons. This explains why the Amazon rainforest has not gotten any larger than it was one thousand years ago.
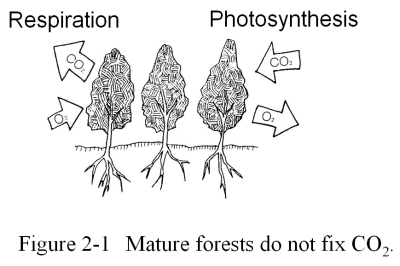
The reason that protecting the world's forests is important from the point of view of the global warming problem is not because of an ability of forests to fix the CO2 that is accumulating in the atmosphere. Rather we must preserve forests because the felling of trees would add the carbon that has been stored in the forests to the atmospheric CO2 pool. It is wrong to believe that if there are forests then the CO2 in the atmosphere will be removed through photosynthesis.
Circulation of materials in the Earth's biosphereIt seems that recently we hear the word "biosphere" used in the media more and more. "Biosphere" is defined as the part of the Earth's surface where the activities of living things take place, including those of humans. The Earth's biosphere is approximately twenty kilometers thick, between the peak of Mt. Everest and Challenger Deep, the deepest abyss of the ocean. When we consider that the diameter of the Earth is roughly 6,000 kilometers, it is clear that the biosphere occupies only a very thin layer of the Earth's surface. If you were to draw a cross-sectional view of the Earth with a compass on this page, no matter how sharp you made the lead of the pencil, the line that you drew will be thicker than the proportional thickness of the biosphere. All life on Earth takes place in this single thin layer of the Earth's surface.
In the same way that we have seen with tropical forests, all mature forests in the world have balanced carbon budgets, that is to say, they release all of the CO2 that they fix by photosynthesis. While we do see some year to year variations caused by droughts, excessive rainfall, accidents, or forest fires, we can say that over a sufficiently long time span, such as decades or more, there is no overall increase in the size of a mature forest and therefore no overall uptake of CO2 from the atmosphere.
Let us take a look at some of the dynamic cycles of materials in the biosphere.
The Soil of a Tropical Forest is Only 20 cm ThickTerrestrial plant life undergoes a cycle that begins with photosynthesis and the growth of leaves and branches, which later fall to the ground and decay forming the organic matter in the soil of the forest floor. The depth of the soil is determined by the rate at which leaves and branches fall to the ground each year and the time it takes for them to decompose and return to the atmosphere in the form of CO2.
In the cold arctic, there are many evergreen trees. It takes a few years before they shed their leaves, and thus the rate at which the leaves fall to the forest floor is slow. Even deciduous trees in cold climates lose only a small number of leaves per year because they photosynthesize slowly. However, in such a cold environment, the rate at which the fallen leaves decay is also slow. Decomposition of organic matter is a process that is strongly controlled by temperature. A decrease in temperature dramatically slows the rate of decomposition. Consider the vegetables and fruits in your refrigerator. They do not rot easily. It takes about 50 years for half of the leaves and branches in cold climates to completely decompose into CO2. As a rule of thumb, we can estimate that the total amount of leaves that will accumulate on the forest floor is the amount that falls from the trees in about twice the time it takes for them to decay, or about one hundred years for cold artic climates. Therefore, in the soil of those regions there is a deep layer of peat, which is just slowly decaying leaves and branches.
By contrast, in the tropics and other warm climates, photosynthesis proceeds much more rapidly, trees become dense with leaves, and consequently the rate at which the leaves fall is also large. The rate of decomposition of the leaves in the soil is also more rapid due to the high temperature. Think of vegetables that were left out of the refrigerator in the summer. In the tropics, it takes less than three years for half of the fallen leaves to decompose and return to the atmosphere as CO2. As a result, the soil layer of tropical forests is comparatively thin because its content only amounts to the volume of fallen leaves and branches accumulated for about five or six years. For example, the soil of a tropical forest in Thailand is reported to be only ten to twenty centimeters thick. One reason why it is difficult for tropical forests to regrow once they have been cut down is the thinness of the soil layer. A rain storm could easily wash away all of the soil, and of course it is very difficult for a forest to grow back without soil.
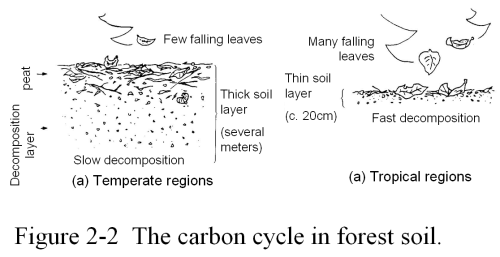
The temperate zone, the region where most of us live, displays soil characteristics that are between those of the tropics and the artic regions. The soil layer is thicker than that found in the tropics; however, unlike the peat that is found in the arctic soil, the soil in the temperate zone is sufficiently decomposed. Thus, the temperate zone possesses the best conditions for the stable growth of plant life. It is clear that even for soil, which one might not think was related to the Sun's energy in any way, the basic characteristics are determined through a dynamic cycle of various materials that is driven by solar energy in the form of photosynthesis and the temperature controlled rate of decomposition.
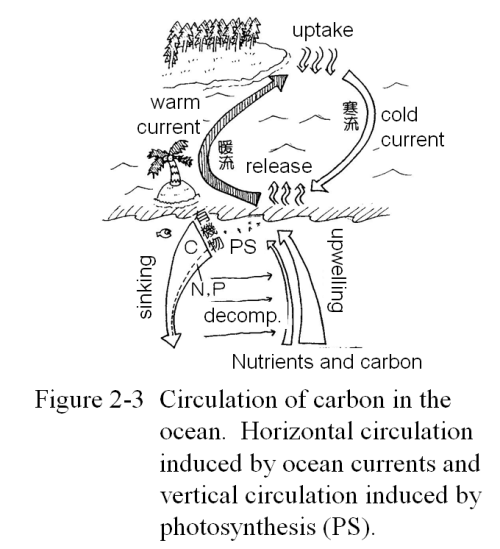
Cycles of Materials in the OceanIn the ocean is a circulation of materials that is just as dynamic as that of the terrestrial ecosystems. Near the sea surface, warm currents such as the Gulf Stream and the Kuroshio travel from the equator to the poles on the western boundaries of the oceans, and cold currents travel from the poles to the equator on the eastern ocean boundaries or the western boundaries of the continents. These currents transport chemical materials throughout the ocean. In the Polar Regions, the seawater temperature is low, and so the solubility of CO2 is high. Consequently, the seawater in these regions take up a large amount of CO2 from the atmosphere. The resultant state of the seawater is like that of a well-chilled beer. There is a great deal of CO2 contained in the liquid, but you do not see any bubbles forming or other evidence that the CO2 is escaping from the liquid. This CO2 saturated polar seawater is carried by a cold current towards the equator. The warm tropical air heats the seawater, causing the solubility of CO2 to decrease. CO2 is released from the warmed seawater back to the atmosphere. The seawater then returns to the Polar Region as a warm current where it is cooled and absorbs CO2 once again. This uptake and release of CO2 and other gases caused by the differences in seawater temperature is the essential mechanism of the material cycles that are caused by ocean currents near the sea surface.
There is also a vertical circulation of materials in the ocean between surface waters and deep waters. The driving force of this circulation is photosynthesis by phytoplankton.
Phytoplankton live out their lives by photosynthetically producing hydrocarbons in the part of the ocean that is exposed to sunlight, a shallow region at the sea surface up to a depth of around 100 meters. Phytoplankton are the base of a food chain whereby phytoplankton are eaten by zooplankton, zooplankton are eaten by small fish, small fish are eaten by larger fish, and so on. However, a large portion of phytoplankton simply die without being eaten. In any case, whether eaten or not, a significant amount of all of the organic material that phytoplankton produce through photosynthesis ultimately sinks to the bottom of the ocean. While sinking, part of this organic material dissolves in seawater and part is decomposed by bacteria. The dead carcasses of plankton and fish as well as fecal pellets and fish scales are decomposed first into a dissolved organic form such as organic acids, and this dissolved organic material is then oxidized by the oxygen contained in the water into CO2. As seawater is weakly alkaline, CO2 is taken into solution as bicarbonate ions. The deeper one goes into the ocean, the higher the concentration of inorganic carbon (e.g. the sum of CO2 and bicarbonate ions) becomes. In places where seawater flows upward, such as near the equator, this inorganic carbon is transported from the deep ocean back toward the sea surface by these upwelling currents. We can summarize the vertical carbon circulation in the ocean as follows: particles of organic carbon, including plankton, are produced in the surface ocean and sink towards the bottom; in the opposite direction, inorganic carbon dissolves or is transported - "upwelled" - toward the surface.
There is another point that requires mention in regard to the vertical carbon cycle in the ocean: the circulation of essential elements other than carbon needed to produce the amino acids and proteins necessary for plant life. The main elements of living matter besides carbon, oxygen and hydrogen are nitrogen and phosphorous. When marine biologists speak of "nutrients for phytoplankton growth", they are usually referring to compounds based on these elements. Furthermore, it is generally agreed that in the well-lit surface waters of the ocean, the rate of photosynthesis is limited by the availability of nitrogen and phosphorous in the sea surface, rather than the availability of carbon, oxygen, or hydrogen. Nitrogen and phosphorous must exist in a "fixed" molecular form that can be taken up by the phytoplankton, such as ammonium and phosphate. Terrestrial plants also require fixed forms of nitrogen and phosphorous, which is the reason that farmers need to add ammonia or other forms of fixed nitrogen to their fields.
Because phytoplankton are so good at using up nutrients, surface waters in most of the open ocean suffer from a lack of nutrients, a state called "oligotrophy". This means that the pace at which phytoplankton reproduce can be accelerated if fertilizers containing nitrogen or phosphorous are supplied. One example is the outbreak of red tides in coastal areas where there are large supplies of fixed nitrogen and phosphorous in the form of agricultural and waste water runoff.
In the open ocean there are no land masses and therefore no runoffs to supply nutrients. From where are the nutrients supplied? You may have guessed the answer: from the deep ocean waters, the same place from which inorganic carbon is supplied in the vertical carbon cycle that we described earlier. When phytoplankton transform CO2 into organic material by photosynthesis, they take in nitrogen and phosphorous at the same time. These nutrients form part of the organic matter of the phytoplankton. When the plankton die and sink out of the surface ocean, the organic nitrogen and phosphorous are gradually oxidized and returned to inorganic nutrient form in the same way as we saw with the organic carbon. This is the reason why there is a higher concentration of nutrients in the deep ocean and why deep ocean waters act as a source of nutrients.
While the majority of the surface open ocean suffers from a shortage of nutrients, areas where deep waters flow upwards or "up-well" to the sea surface are rich in nutrients. In these regions, phytoplankton proliferate, the food chain is well supplied, and a great variety of fish can be seen. Indeed, many of the best fishing grounds are situated in such areas.
Figure 2-3 shows the material cycle in the ocean driven by photosynthesis.
The Cycle of Materials in the Atmosphere and the Tragedy of Chlorofluorocarbons
The cycling of materials in the atmosphere is also dynamic. For example, the methane that is released from swamps, rice fields, and "cattle burps" will be oxidized to CO2 in an average of seven years after being released into the atmosphere.
Chlorofluorocarbons (CFCs) are chemicals that were widely used in sprays, air conditioning, dry cleaners, and many other applications. CFC's possess many excellent features, one of the most important being that they are chemically stable. Because they hardly react at all with other substances, they do not burn, corrode, or cause toxic reactions to the human body. Thus they were thought to be safe and harmless. However, because CFC's are so resistant to oxidation, they can spread all the way into the stratosphere without being broken down. In the stratosphere, there exists a thin layer of ozone. Ozone is a molecular form of oxygen which is made of three oxygen atoms, written in chemical shorthand as "O3".
Ultraviolet (UV) rays from the Sun radiate onto the stratosphere. UV rays contain intense photon energy which is why they are so harmful to living beings. These rays have so much energy that they cause normal oxygen molecules (O2) to react and produce ozone (O3). The UV rays are consumed by this photo-reaction process. Therefore, the ozone layer protects us from the damaging effect of UV rays by absorbing them before they reach the earth's surface. Because ozone consists of three oxygen atoms - one more oxygen atom than stable oxygen molecules - it is an extremely powerful oxidizing agent. While CFC's do not react with oxygen and thus are able to enter the stratosphere intact, they do react with ozone. Because the compounds that are produced when the CFC's react with ozone act as catalysts to consume even more ozone, each CFC molecule is able to destroy on average over 50 molecules of ozone. Therefore, a small amount of CFC's is capable of severely depleting the stratosphere of ozone.
Through this mechanism, CFC's are destroying the ozone layer. Thus the story of one of the most miraculous man-made chemicals has ended in tragedy. However, in understanding the mechanism behind the destruction of the ozone layer, it has been possible to work out a countermeasure. Researchers have developed replacements for CFC's, which are still basically chemically stable but can be oxidized by oxygen within a time period of about ten years. Ten years is quick enough to stop the CFC replacements from reaching the stratosphere and destroying the ozone there.
So far, we have discussed the dynamic circulation systems that exist on land, in the ocean and the atmosphere. The mechanisms that bring about these circulations are, as we described above, photosynthesis, ocean currents, and winds. With the Sun's energy as its basic driving force, carbon circulates in the biosphere while constantly changing its chemical composition.
Missing SinkPrior to the emergence of human civilization, the land, ocean, and atmosphere were in a state of what we can call dynamic equilibrium. In other words, a certain fixed amount of carbon was circulating dynamically in the biosphere, but the total amount of carbon was not changing significantly. The dynamic carbon cycle stayed in this constant, balanced state until about 5,000 years ago. The ocean absorbed enough CO2 so that the concentration of CO2 in the ocean was in equilibrium with that in the atmosphere. Where the climate was favorable, plant life flourished. Under these stable conditions, the CO2 concentration in the atmosphere was a nearly constant 275 ppm. Even when the concentration of atmospheric CO2 began to rise in response to human activities, most of the excessive CO2 dissolved into the oceans, and the remainder was taken up by plants through slightly increased rates of photosynthesis in the terrestrial biosphere. Thus the concentration of CO2 in the atmosphere was maintained at the level of 275 ppm.
The development of civilization has been accompanied by expansion of farm land and the felling of large numbers of trees for firewood. As we saw before, this deforestation results in an additional release of carbon from the land. Moreover, the mining and burning of fossil fuels has added carbon to the atmosphere which, prior to being brought out of the ground, had remained buried and thus uninvolved in the dynamic global carbon cycle. These human activities are the main two factors that have contributed to the recent CO2 increase in the atmosphere.
Figure 2-4 shows a recent estimate of the "budget" of carbon in the biosphere, i.e. the total amounts of carbon coming in and going out of the biosphere each year. Almost all carbon in the atmosphere takes the form of CO2. If we were to weigh all of the carbon in all of the CO2 in the atmosphere, we would find that the total weight of this atmospheric carbon is approximately 700,000,000,000 (7 billion) tons. The carbon content on land is about three times higher than in the atmosphere; that carbon is contained mainly in plant life and organic material in the soils. The amount of carbon in the oceans is about 60 times as much as that in the atmosphere, and that carbon exists primarily as bicarbonate ions.
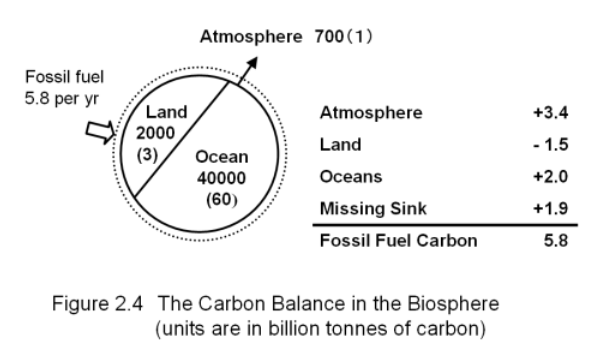
In 1990, the carbon emission caused by the combustion of fossil fuels by humans totaled 5,800,000,000 (5.8 billion) tons. This emission of carbon caused the CO2 concentration in the atmosphere to rise by 1.6 ppm.
The oceans absorb a part of the CO2 that is emitted into the atmosphere, but it is difficult to measure directly how much. The reason is because, unlike in the atmosphere, carbon is unevenly distributed in oceans. An increase in atmospheric CO2 concentration causes the ocean water CO2 concentration1 to rise only in the surface layer of the ocean, leaving the deeper ocean waters unaffected. This phenomenon is further complicated by the vertical carbon circulation in the oceans that we described earlier. Consequently, the CO2 concentration in the ocean changes greatly with depth. Moreover, there are even variations in the concentration of CO2 in different areas of the surface ocean. It would be tremendously difficult to monitor a sufficient number of observation points to enable an accurate measurement of the total CO2 content in all the oceans of the Earth. However, one way to estimate the total amount of CO2 absorbed by the oceans is to construct a computer model or simulation based on our understanding of the mechanism by which oceans take in atmospheric CO2. The estimate thus obtained can be compared with the actual CO2 concentrations measured in a limited number of locations in the ocean in order to examine in a statistical way how adequate the model is. By using this method, scientists have estimated that the total amount of CO2 that is absorbed by all the oceans on the Earth each year is at most two billion tons.
Therefore, we can estimate that the total amount of CO2 that is accumulating in the atmosphere and the oceans is 5.4 billion tons . As the annual CO2 emissions produced by the combustion of fossil fuels is estimated to be about 5.8 billion tons, 0.4 billion tons of carbon appears to be left unaccounted. This carbon must be absorbed by the land because the biosphere is comprised only of the atmosphere, the oceans, and the land. Therefore, until very recently, many researchers believed that the terrestrial biosphere, such as forests, fixed this additional carbon by means of photosynthesis. However, if we look at the situation more closely, it is clear that the terrestrial biosphere cannot be fixing carbon. As we discussed before, any CO2 that is fixed in mature forests is matched by an equivalent release of CO2 through decay. Furthermore, due to deforestation, tropical forests are certainly shrinking, resulting in the net release of CO2 to the atmosphere. In fact, the best estimate is that at least 1.5 billion tons of carbon is being released from the land each year. Adding this amount to the 0.4 billion tons left over from burning fossil fuels, we have 1.9 billion tons of carbon that is unaccounted; we do not know where this carbon is going.
1.9 billion tons is an enormous amount of carbon. For example, the total amount of steel produced worldwide is about 800 million tons, which is less than a half of the "missing" carbon. But this carbon cannot just disappear. It cannot change into another chemical element such as nitrogen without a nuclear reaction. It cannot escape into the space, nor can it go underground. This carbon must be accumulating somewhere, but no one knows where. This problem is referred to as the "missing sink" and is one of the biggest mysteries in the earth sciences.
It is the dismal truth that today's most advanced knowledge about the circulation of the different elements in the earth is unable to account for this 1.9 billion tons of carbon per year. In fact, in the 1990's the rate of the increase of CO2 concentration in the atmosphere slowed down. This is even more confusing, because during that time it appears that as much as 3.4 billion tons of carbon, the amount of carbon that until now has been accumulating in the atmosphere each year, was taken up by the missing sink.
Without doubt, there are many things that we do not know about the cycling of carbon in the Earth's biosphere. But what is evident is the overall mechanism of the carbon cycle. We have seen clearly that (a) CO2 emitted from the combustion of fossil fuels and the cutting down of forests does not vanish but must be accumulated in the atmosphere, the oceans, and the land, and (b) 40% of the carbon produced by the combustion of fossil fuels is stored in the atmosphere as evidenced by to observational data gathered over a long period of time. These are indisputable scientific facts.
The Response Rate of the EarthFor thousands of years prior to the Industrial Revolution, the CO2 concentration in the atmosphere stayed essentially constant at 275 ppm. However, since the Industrial Revolution, which began with the large-scale use of the steam engine, humanity has gained energy by burning fossil fuels at the expense of extensive emission of CO2.
Were there any CO2-producing human activities before the Industrial Revolution? Actually, humans have been cutting down trees throughout history, and this destruction of forests has long been a source of CO2. Mesopotamia, once a rich forest region, was transformed into a desert due to the development of agricultural land and damage to the soil by seawater. In China, the construction of the Great Wall carried out during the rule of the Shin Empire (B. C. 221 - 210) required a large supply of fuel to bake the innumerable bricks used in the wall. Extensive forest area was cut down to supply the fuel for this project, and some people believe that this deforestation brought about a desertification of that region. At one time, Europe also exploited its forests for fuel to produce steel, turning bountiful forests into wastelands. Thus, it is clear that the development of civilization was accompanied by deforestation. Let us try to estimate the total amount of CO2 emission resulting from this deforestation.
In forests, carbon is contained in trees and plants above the ground and in roots and the organic matter in soil under the ground. By contrast, very little carbon exists in deserts. Thus, we can conclude that if desertification occurs, in other words if a forest is transformed into a desert, most of the carbon which was stored in that forest will be transferred to the atmosphere.
For example, imagine that the entire area of the Sahara Desert was covered by a huge forest. It has been estimated that the quantity of carbon that would be accumulated there would be 180 billion tons, which is thirty times that of the annual carbon release from the use of fossil fuels. One can imagine what a huge amount of carbon was removed from the terrestrial biosphere due to the desertification in China and Mesopotamia. Furthermore, even the transformation of forests, woods, and grasslands into farms results in CO2 being moved from the terrestrial biosphere to the atmosphere, because although farms contain some carbon in the form of the agricultural products, this amount is much smaller than the amount contained in a forest of the same area.
The total carbon emission caused by deforestation throughout the entire history of humanity is unknown because of the lack of data on the earlier historical periods. However, as the total carbon emission is thought to be about 300 billion tons since the onset of the Industrial Revolution. This is a considerable quantity of carbon, almost half the present volume of atmospheric carbon, which is about 700 billion tons. Meanwhile, the combustion of fossil fuels has so far produced about 170 billion tons of carbon, just a little more than half of the total released by deforestation.
Why is it that the concentration of CO2 in the atmosphere before the Industrial Revolution stayed at the level of 275 ppm, even though almost twice as much carbon was released prior to the Industrial Revolution than after? The reason is the difference in the speed at which the carbon was released. Deforestation proceeded slowly over a stretch of several centuries preceding the Industrial Revolution. When carbon is released gradually into the atmosphere, it can be easily absorbed by oceans. Let us take another look at Figure 2-4. The distribution of carbon in the atmosphere, the land, and the oceans is 1 to 3 to 60. We have seen that when carbon moves through the biosphere, it goes through various cycles such as photosynthesis and the warming of seawater that are driven mainly by the sun's energy. These cycles cause the carbon to be distributed between the atmosphere, the land, and the oceans in this proportion. Therefore, it is easy to see that when the rate of carbon emission is slow enough the oceans will absorb most of the carbon, about 60 times more than the amount that remains in the atmosphere. But it takes time to reach this state of equilibrium.
How Fast Do Ocean Waters Mix?"Sloth-like" is an appropriate word for describing the pace at which ocean waters mix. The mixing occurs both vertically and horizontally. We discussed the warm and cold currents that mix the ocean horizontally. Of concern here is the question of how fast the vertical mixing takes place. The entire ocean will have absorbed its proportion of carbon from the biosphere only when the vertical mixing is completed. Before this vertical mixing happens, only the surface water will be in equilibrium with the land and the atmosphere in terms of carbon content.
There are two mechanisms which cause ocean waters to mix vertically. The first concerns the movements of vertical currents, although the details regarding the mechanism are yet to be clarified. Descending currents generally occur near the poles where surface waters cool, become more dense and sink under the warmer deep waters (e.g. there is a large descending current in the Spitzbergen Offing in northern England). Ascending currents occur near the equator and constitute the upwelling of deep ocean waters that we mentioned earlier in our discussion of the supply of plant nutrients to the open ocean. In the Pacific Ocean, the average speed of the ascending currents has been estimated to be about two meters per year. Because the average depth of the ocean is on the order of 3,000 meters, it would take about 1,500 years to complete one full vertical circulation of the entire Pacific Ocean.
The second mechanism concerns local currents, which occur in the ocean as random 'eddies'. Eddies of different sizes exist in the ocean, and they play a key role in the mixing process by dispersing the ocean waters. The dispersion that occurs due to these eddies is usually expressed as a coefficient with units of distance squared per time2. The coefficient of dispersion in the Pacific Ocean is about 2,000 meters squared per year. The time needed to mix water by dispersion over a certain depth is approximately equal to the depth squared - remember we are dealing with areas! - divided by this coefficient of dispersion. For a depth of 3,000 meters, the time required is about 4,500 years.
We can determine the time needed to mix the oceans vertically using both mechanisms. Since the times that we calculated are just the inverses of the rates of mixing of the entire ocean per unit time, the rate of mixing by both mechanisms is (one mixing per 1500 years) plus (one mixing per 4500 years) or (four mixings per 4500 years). Therefore, the actual amount of time needed to mix the ocean once is more than one thousand years. At least this much time is necessary for the carbon content in the whole ocean to come into equilibrium with the CO2 concentration in the atmosphere.
Global Warming is a Problem of TodaySince the dawn of history, we have seen that humanity has released massive amounts of CO2 by deforestation. However, until the last century the release was done at a very slow pace, and thus the oceans could absorb most of this CO2. As a consequence, the CO2 concentration in the atmosphere stayed at around 275 ppm.
After the Industrial Revolution, the situation changed drastically. Fossil fuels which had been lying under the ground and thus were uninvolved in the cycle of carbon in the biosphere were extracted by human industry and burned to produce energy. As a result, the carbon that had previously been locked away underground became introduced into the biosphere - mainly as CO2 released into the atmosphere. When the CO2 produced from this combustion is added to the carbon released by deforestation, we find that about 1% of the total carbon content of the atmosphere is being emitted annually. This means that the carbon emitted over the span of one hundred years will be equal to the total amount of carbon in the atmosphere. The oceans cannot possibly keep up with this rate of CO2 increase. It is for this reason that the sudden increase in the CO2 concentration in the atmosphere has begun.
In chapter one we discussed how some people have suggested that the CO2 problem can be considered a political problem while others argue that it is a population problem, and still others say that it is a problem between developed countries of the Northern Hemisphere and developing countries of the South. It is true that each of these claims highlights one aspect of this many-sided problem. But the underlying scientific cause of the global warming problem is the increasing CO2 concentration in the atmosphere. Atmospheric CO2 concentration is a very basic indicator of the health of our planet's ecological system. Today's rate of carbon emission, 1% of the total carbon content in the entire atmosphere per year, exceeds by far the rate that the oceans can uptake this carbon. As the oceans are the largest storage of carbon in the Earth's biosphere, it is this outstripping of the oceans' absorption ability that is the essence of the CO2 problem.
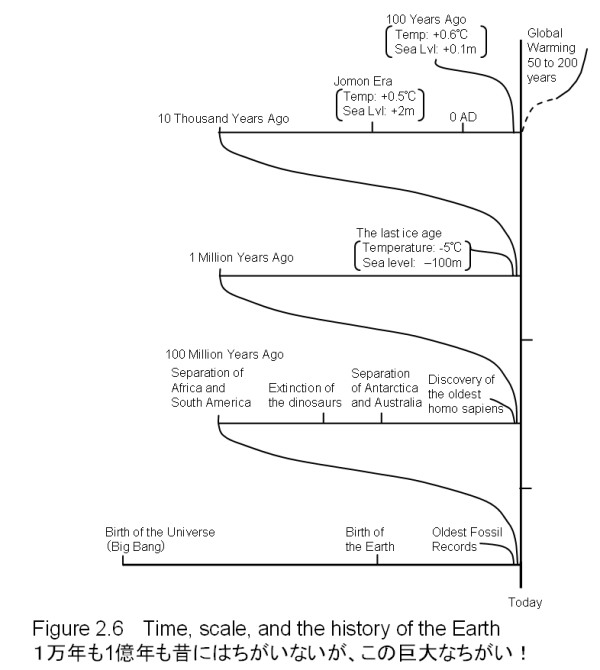
100 Million Years is 100 Years Multiplied by One MillionThe most ancient civilization is believed to have begun about nine thousand years ago in Central Asia. What we have discussed so far covers the period since that time. Up to the present day, history has witnessed the rise and fall of countless civilizations: Mesopotamia, the Hwang Ho (the Yellow River), the dynasties of China, the Greeks, and the Roman Empire. Nine thousand years is an incredibly long stretch of time. Compared to this long period of time, the few decades needed for the CO2 concentration in the atmosphere to double is like an instant. This difference in time scale is what leads us to conclude that the current change in the atmospheric concentration of CO2 is quite abnormal.
Of course, these nine thousand years of the history of civilization are but a flash in the long history of the Earth. Some people claim that global changes are nothing to worry about by using the following argument. "The Earth has experienced many changes. Even before the era of dinosaurs and the Carboniferous period, there was a time when the temperature of the Earth was several degrees higher than that of the present day, and there were also Ice Ages. Global warming can be considered as just one of these kinds of events". But we must be cautious of this kind of argument and properly understand its danger.
We do not deny that the Earth has undergone major changes, but these changes normally occur over enormously long stretches of time. A person's life span is less than one hundred years; one hundred human life spans is ten thousand years, which we have seen is approximately the length of the history of human civilization. Take five hundred of these time spans and we get five million years; this is the approximate length of time that human beings have existed as a species. In other words, in the time that homo sapiens have existed on the earth, the rise of human civilization to today could be repeated five hundred times. Dinosaurs are said to have become extinct sixty-five million years ago, which is thirteen of the five million year lengths of time that humans have walked the earth. The Carboniferous period dates back to two hundred and fifty million years ago, which is sixty-five million years times four. Thus the Carboniferous period occurred four times the length of time since the dinosaurs lived on the Earth. The period of time in which the Earth experiences normal changes is truly incomprehensible. A hundred million years is one hundred years times one million - who can possibly imagine a period of time that is one million times longer than one's own lifespan?
So many things must have happened within this vast, unimaginable length of time. Scientists have shown that the global average temperature fluctuated over a range of 10 degrees Celsius, and the sea level rose and fell several hundred meters over this span of time. Even the continents have moved. In fact, the world geography of millions of years ago does not bear the slightest resemblance to today's continents. From the mid-twentieth century onwards, our understanding of this transformation of land masses and oceans has changed greatly. The development of this "modern" understanding was triggered by a man named Alfred Wegener. Wegener noticed that when the outlines of today's continents were merged together, one huge continent resulted. Based on this finding, he published a book called "Die Einstehung der Kontinente und Ozeane (The Origin of the Continents and the Seas)" in 1915, in which he stated that all the continents existing today were originally one single land mass. At the time of publication, his theory was fiercely criticized, especially by the American academic society, and it did not receive wide acceptance until the middle of this century. Now, Wegener's theory is believed to be true, and it has contributed largely to a dramatic alteration in our ideas about the land and the ocean.
According to Wegener's theory, two hundred million years ago, Earth's continents once formed a single continent called the Pangea Continent, which is shown in Figure 2-5. The Pangea Continent then split up into the Rhodesia Continent and the Gondowana Continent which, over an enormous span of time, split further to form the surface of the Earth as we see in present world atlases.
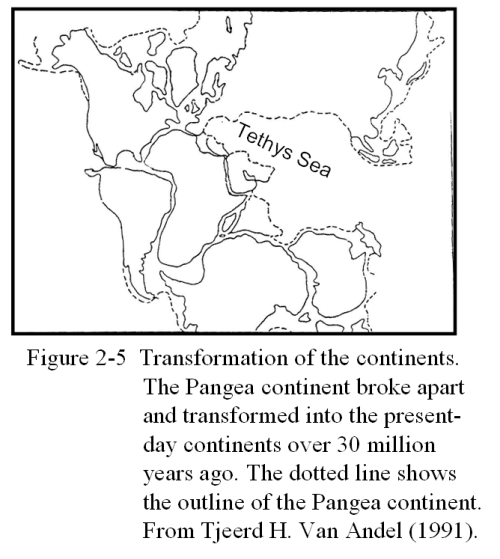
These processes by which the continents split and moved must have taken ten million to one hundred million years. No animal could have been aware of such processes actually taking place during the span of its life time, for its life merely appears as an instant against the slow pace of the continental changes. Not only the animal itself but also uncountable descendents must have lived out their lives, whole species flourished and gone extinct without the awareness of such changes. These slow changes are in sharp contrast to the sudden coolings of the Earth that were caused by the eruptions of massive volcanoes such as Mt. Asama and Mt. Pinatubo, which we discussed in Chapter One. These near instantaneous events must have caused critical changes in ecosystems and living beings.
However, most past geological events are unknown due to the lack of data. For example, it seems certain that ice ages occur on the Earth periodically. The current theory states that the ice ages can be explained by periodic shifts in the Earth's revolution around the Sun as well as the Earth's rotation about its own axis. But there is no decisive proof for this theory, and in fact, many other theories have been put forward. Theories describing the extinction of dinosaurs are various, ranging from the impact of a giant meteorite, to natural selection by mammals, to climatic changes caused by the creation of mountains. Furthermore, there is nothing definite about the causal relation between each of these independent events.
What we know for sure is that many catastrophic events have taken place within the long period of the Earth's history. If a comet or meteorite similar to the one that collided with Jupiter in July 1994 hits our planet, it will have tremendously destructive consequences for all life on Earth, including humans. But the chance of encountering such an event during one's lifetime or even the lifetime of a civilization is immeasurably small; it occurs only once every several million years. Thus, we should not worry about events such as the immediate collision of a comet, though the Earth must have already experienced such collisions a few times since it was formed.
Global Warming is Only 100 Years OldWe cannot sense geological changes, such as the creation of mountains, which occur as slowly as the history of the Earth's development. It is pointless to worry about a catastrophic event which supposedly occurs once in a million years. Nevertheless, it is wrong to argue that global warming can also be ignored because the Earth has gone through similar changes in the past. The reason why we must be concerned about global warming is that it poses to us a potential danger on a time scale that is of the same order as the time scale of our lives, an instant when compared to geological changes such as ice ages and mountain formation. If we leave the CO2 problem unaddressed, global warming will continue to progress during our life times. Our children and grandchildren will almost certainly experience even more critical conditions, as it is predicted that the effects of global warming will be greatly accelerated during their lifetimes.
Footnotes:
1Actually the concentration of CO2, bicarbonate, and carbonate ions but we will refer to the total as the CO2 concentration in the ocean.
2While speed, having units of distance per time, is the distance covered by an object in a certain amount of time, dispersion can be thought of as the amount of area over which some material spreads in a given amount of time.
